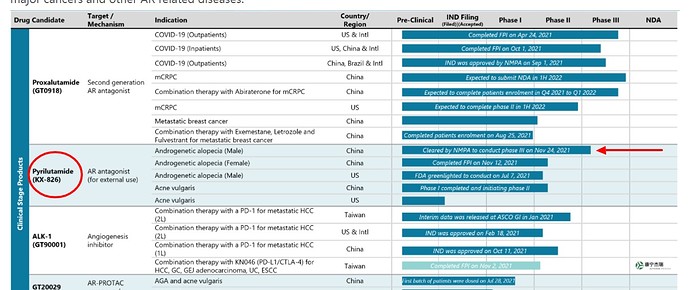
Kintor announced that the primary endpoint for phase II clinical trial of pyrilutamide (“KX-826”, tincture) for the treatment of androgenetic alopecia or pattern baldness was met and the results were “statistically significant and clinically meaningful”, according to one of the press releases for Kintor.
Kintor Pharma’s pyrilutamide is a topical androgen receptor inhibitor that targets hair follicle
sebaceous glands…
Kintor’s phase II trial was conducted and concluded in China. Separately, Kintor also received FDA approval to conduct Phase II trial in the US according to Bloomberg news.
The primary endpoint for the phase II trial was established to evaluate the response rate or the efficacy of KX-826 for the treatment of androgenetic alopecia. It measured the change from baseline in hair counts at week 24 in comparison with the controlled group.
Trial result details not available at this point.
Phase III trial is scheduled to begin in China in Q4, 2021.
More from this article below
Kintor Pharma Announces the Primary Endpoint of Phase II Clinical Study for KX-826’s Treatment of Androgenetic Alopecia Was Met
Hasson & Wong sponsored post - presently a Top 3 Hair Clinic of ALL TIME worldwide according to HairSite patient result statistics.
Schedule a FREE consultation to see if you are a good candidate for FUE hair transplant and other advanced hair restoration treatments.