Medical Review & Authorship
**Primary Author & Treating Physician: Dr. Jinkal Kunjadiya, MD (Dermatology)**Consultant Dermatologist & Hair Transplant SurgeonHairfree Hairgrow Clinic, Surat, India
This article is based on a real clinical case and has been medically reviewed for accuracy, patient safety, and ethical compliance.
Medical Disclaimer
This case study is provided for educational purposes only. Individual results may vary based on genetic factors, scalp condition, and adherence to post-treatment care. Hair transplant outcomes cannot be guaranteed. This content does not replace professional medical consultation.
Introduction
Androgenetic alopecia is a progressive, genetically determined condition that affects a significant proportion of adult males. While medical therapies such as minoxidil and DHT blockers can slow progression, they often fail to restore lost hair in advanced stages. Surgical hair transplantation, when performed by trained dermatologists using evidence-based techniques, offers predictable and long-term cosmetic improvement.
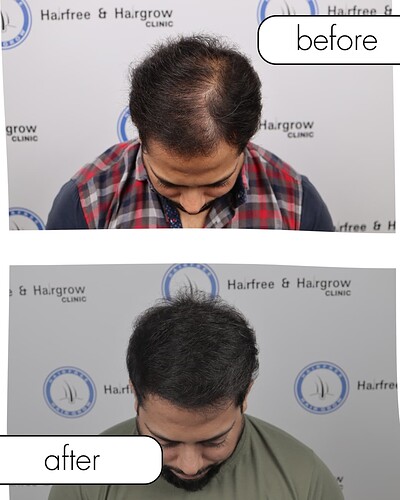
This case study documents the complete clinical journey of a patient treated with I-FUE hair transplantation, supported by platelet-rich plasma (PRP) therapy and standardized medical management.
Patient Information (De-Identified for Privacy)
-
Initials: S.P.
-
Age: 37 years
-
Gender: Male
-
Location: Gujarat, India
Patient consent was obtained for clinical documentation and photographic use.
Diagnosis & Clinical Assessment
Primary Diagnosis
-
Androgenetic alopecia (male pattern hair loss)
-
Norwood–Hamilton Grade III–IV
Duration & Progression
-
Hair fall duration: ~4–5 years
-
Noticeable frontal baldness: ~2–3 years
-
Pattern observed:
-
Complete frontal hairline recession
-
Diffuse thinning of frontal and mid-scalp regions
-
Associated Scalp Findings
-
Mild pruritus (itching)
-
Occasional inflammatory follicular lesions
Genetic & Medical Risk Assessment
-
Family history: Positive maternal history of baldness
-
Comorbidities: None reported
-
Systemic health: No diabetes, hypertension, asthma, or autoimmune disease
-
Keloid tendency: Negative
-
Allergy history: No known drug or food allergies
The patient was deemed a suitable candidate for surgical hair restoration based on donor availability and scalp health.
Prior Non-Surgical Management
Before surgery, the patient had used:
-
Topical Minoxidil
-
Nutritional supplements
These interventions provided limited and temporary improvement, consistent with advanced androgenetic alopecia.
Clinical Decision-Making & Informed Consent
A detailed consultation was conducted, covering:
-
Disease progression
-
Surgical and non-surgical options
-
Realistic expectations
-
Possible risks and complications
-
Long-term maintenance requirements
The patient provided written informed consent before the procedure.
Surgical Methodology (Evidence-Based)
Preoperative Planning
-
Age-appropriate, natural hairline design
-
Restoration of the frontal hairline and temples
-
Density enhancement in the mid-scalp region
Trichoscopic evaluation confirmed adequate donor density and hair caliber.
Anesthesia & Safety Measures
-
Local modified block anesthesia
-
Pre-procedure sensitivity testing
-
Continuous patient monitoring
Recipient Site Creation
-
Metal blades used:
-
1.2 mm for frontal hairline
-
1.4 mm for other areas
-
-
Slits aligned with natural hair angulation to prevent unnatural growth patterns
Donor Harvesting
-
Micro-FUE technique
-
0.8 mm sterile titanium punch
-
Magnification-assisted extraction
Graft composition:
-
~20% single-hair units
-
~80% multi-hair units
-
High proportion of triple-hair grafts, improving density outcomes
Grafts were preserved in chilled Ringer’s Lactate solution to maintain follicular viability.
Graft Implantation
-
Performed using the K.E.E.P. implanter
-
Reduced handling trauma
-
Even graft distribution
Post-Procedure Care & Monitoring
-
Standard postoperative medications were prescribed
-
Written aftercare protocol provided
-
Direct physician access ensured for early complication management
Post-Transplant Hair Growth Timeline
Shedding Phase (0–2 Months)
-
Expected telogen shedding of transplanted hairs (~70%)
-
Two PRP sessions were administered
-
Native hair fall reduced
Early Regrowth (3–5 Months)
-
New hair emergence observed
-
Third PRP session administered
-
Visible improvement in scalp coverage
Active Growth (6–9 Months)
-
~80% graft survival visible
-
Hair styling possible
-
Booster PRP at 9 months
Final Outcome (10–12 Months)
-
Stable, natural-appearing density
-
Mature hairline
-
No visible donor scarring
Outcome & Patient-Reported Satisfaction
The patient reported:
-
High satisfaction with density and natural appearance
-
Improved confidence
-
No long-term complications
Clinical Discussion (Expert Perspective)
This case supports current dermatological evidence that I-FUE combined with PRP and medical therapy can provide reliable outcomes in moderate androgenetic alopecia when:
-
Patient selection is appropriate.
-
Donor management is conservative.
-
Long-term maintenance is emphasized.
Key Takeaway for Patients
Hair transplantation is a medical procedure, not a cosmetic shortcut. Results depend on genetics, surgical expertise, and adherence to maintenance therapy.
When to Seek Professional Advice
If you are experiencing progressive hair loss:
-
Early evaluation improves outcomes.
-
Medical therapy may delay surgery.
-
Surgical intervention should only be performed by trained dermatologists.
About Hairfree and Hairgrow Clinic
Hairfree and Hairgrow Clinic is India Best Hair Transplant clinic follows ethical medical practices, evidence-based protocols, and patient-centric care under licensed dermatologists.