I received this email from Histogen Inc (guess I’m on a list of people to update for some reason).
There is a press release being published tomorrow that gives the 1 year updated results. I have attached the 2 images that were on the email, and reproduced the text in its entirety…
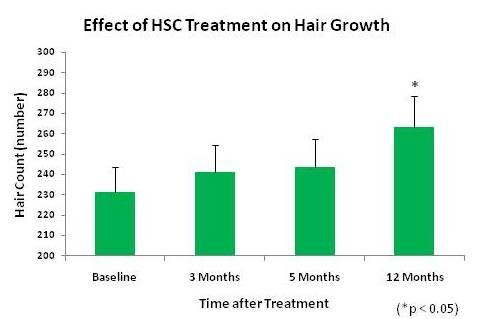
Histogen is excited to share the one year results of our pilot clinical trial of HSC for hair growth, which we will be announcing tomorrow. Please find the news release below. Particularly interesting and exciting that the new growth was not only rapid (significant growth at 3 months), but has continued over time without additional treatments. A statistically significant increase in hair count was seen at the one year follow-up.
Please feel free to share the announcement with friends, family and/or your local news outlets. As always, if I can answer any questions please contact me directly at the email below.
Hope you are well, and look forward to being in touch with further news soon!
Eileen
Eileen Naughton Brandt
Director of Communications
Histogen, Inc.
619.318.7821
PS – You are receiving this email as a member of Histogen’s HSC interest list. If you no longer wish to receive news from Histogen, please reply to this email and we would be happy to remove you from our list.
HSC Trial Shows Continued Significant Hair Growth at One Year Follow-up
Histogen to present one year clinical trial data at Society for Investigative Dermatology Annual Meeting
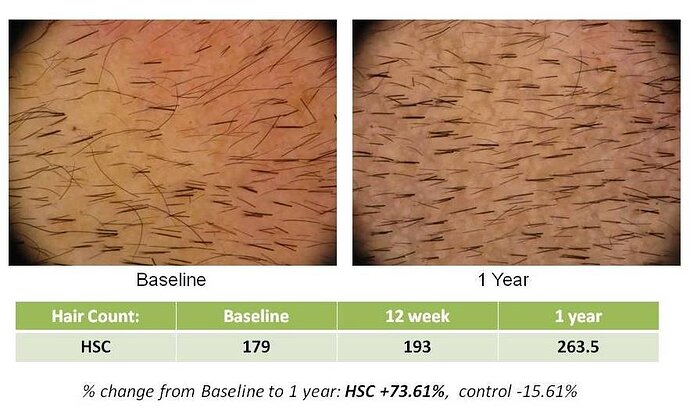
SAN DIEGO, April 13, 2010 – Histogen, Inc., a regenerative medicine company developing solutions based on the products of newborn cells grown under embryonic conditions, today announced the one year data findings of its Hair Stimulating Complex (HSC) pilot clinical trial. Statistically significant new hair growth was seen in HSC-treated subjects at this follow-up timepoint, one year after their single treatment with HSC.
In addition to the number of new hairs, a statistically significant (p<0.05) increase in hair density, which is directly related to hair count over the treatment areas, was also seen at the one-year timepoint. Other efficacy factors, such as hair thickness and terminal hair density, showed an upward trend at this timepoint as well. The pilot trial tested two formulations of HSC and, although one formulation was determined to be superior, significant new hair growth was seen in both groups. (p=0.032)
This new data indicates that a single HSC treatment not only results in rapid hair growth (statistically significant increases in the number of terminal hairs, hair thickness density and hair shaft diameter was seen at three months), but that these results persist over time.
“Seeing continued hair growth at this one year follow-up is truly groundbreaking,” said Dr. Craig Ziering, Founder of Ziering Medical and Principal Investigator on the HSC clinical trial. “Not only do currently available non-surgical treatments show limited hair regrowth, but any new hair is lost shortly after discontinued use. We now have preliminary evidence that HSC significantly increases hair counts, and that the effects of a single treatment are lasting.”
The pilot 24 subject clinical trial of HSC was a double-blind, placebo-controlled evaluation of safety in the clinical application of the product as an injectable for hair growth. Quantitative analysis of clinical macrophotography and subject biopsies were utilized to evaluate treatment safety and efficacy. No adverse events were seen at any timepoint, including the one year follow-up.
HSC is a proprietary formulation of naturally secreted embryonic-like proteins and growth factors. In addition to Wnt 7a, which is recognized to be critical in the induction and maintenance of hair follicle growth, the complex contains a wide variety of factors typically produced by embryonic cells and which are important to the hair cycle, such as follistatin. While these embryonic-like materials are amplified under Histogen’s unique manufacturing conditions, undesirable proteins and growth factors, including Wnt 5a, which has been shown to be associated with cancer, are eliminated.
“The development of hair follicles is the consequence of a complex interplay of factors that is still being unraveled,” said Dr. Jonathan Mansbridge, Histogen’s Chief Scientific Officer. “However, several factors critical to hair growth, such as Wnt 7a, follistatin, VEGF and KGF, are present in HSC, and their secretion by the cells is stimulated by the culture conditions we use. The embryonic conditions under which our cells are manufactured not only upregulates genes associated with hair growth, but induces significantly more production of these critical factors than seen with other culture conditions.”
HSC is a unique composition resulting from growing newborn cells under embryonic conditions. The hypoxia/microgravity results in large amounts of follistatin (41.6 ng/ml in HSC versus 6.75 ng/ml in normal culture conditioned media), VEGF (9.1 ng/ml versus 2 ng/ml), KGF (5.4 ng/ml versus 2.1 ng/ml), and a lack of the scar-related TGF beta (0 ng/ml versus 1.7 ng/ml in normal cultures). This composition is covered by pending US patent #2010/0047305.
.
The full results of the HSC clinical trial will be presented at the Society for Investigative Dermatology (SID) Annual Meeting, taking place in Atlanta, May 5-8, 2010. Presentation abstracts have been published by SID are now available for review at ScholarOne Abstracts - Login.
Histogen is currently in planning stages for the next clinical trial of HSC, which is scheduled to begin in late 2010. This next trial will further examine the safety and efficacy of the HSC product as an injectable for hair growth, and will also evaluate optimum treatment dosing and delivery. Histogen is currently seeking a Series B investment round, which will be utilized to finance these next stages of HSC development and trials.
About Histogen
Histogen, launched in 2007, seeks to redefine regenerative medicine by developing a series of high value products that do not contain embryonic stem cells or animal components. Through Histogen’s proprietary bioreactors that mimic the embryonic environment, newborn cells are encouraged to naturally produce the vital proteins and growth factors from which the Company has developed its rich product portfolio. Histogen has two product families – a proprietary liquid complex of embryonic-like proteins and growth factors, and a human Extracellular Matrix (ECM) material, ExCeltrix.
For more information, please visit http://www.histogen.com.


 Effin Sweet ^^
Effin Sweet ^^