Evaluation of Sexual Function With an International Index of Erectile Function in Subjects Taking Finasteride for Androgenetic Alopecia
Archives of Dermatology
Vol. 140 No. 7, July 2004
INTRODUCTION
Because a systemic treatment for androgenetic alopecia (AGA) is now available, a careful balance between benefits and potential unfavorable consequences is necessary. One of the aspects that has gained increasing attention in this respect is sexual function.
The main adverse effects related to finasteride therapy for AGA involve the sphere of sexual function (loss of libido, erectile dysfunction, and decreased ejaculate volume). In clinical trials for the evaluation of finasteride efficacy, sexual side effects have been reported in 4.4% of patients treated with the active drug and in 2.2% of patients taking placebo,1-3 with 1.4% of patients discontinuing the studies because of sexual side effects in the first year of treatment and 1.3% discontinuing in the extension studies. These adverse effects are less apparent in the clinical setting, where sexual side effects occur in fewer than 0.5% of subjects who take 1 mg of finasteride.4
The abridged 5-item version of the International Index of Erectile Function (IIEF-5) is a cross-culturally and psychometrically valid measure of male sexual function that has been used to measure the efficacy of oral sildenafil citrate and oral phentolamine mesylate in the treatment of erectile dysfunction.5-7 The IIEF-5, which is a brief, reliable self-administered questionnaire consisting of 15 questions, has been linguistically validated in 31 languages, including Italian. It evaluates 5 domains: erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall sexual satisfaction.5-7 The erectile function domain of the IIEF-5 is considered a valid diagnostic tool for distinguishing between men with and without erectile dysfunction. An erectile function score of less than 25 is indicative of erectile dysfunction. The IIEF-5 demonstrates adequate sensitivity and specificity for detecting treatment-related changes in patients with erectile dysfunction.5
In a preliminary study, we showed that the sexual and erectile functions of subjects taking finasteride do not significantly differ from those of age-matched controls.4 The aim of our current study was to evaluate variations in sexual and erectile functions in subjects taking 1 mg of finasteride for AGA by administering the IIEF-5 before and during treatment.
METHODS
In a multicenter study carried out in Italy, the sexual function of a group of 186 patients with AGA was evaluated before and 4 to 6 months after the initiation of finasteride therapy (1 mg). All patients (age range, 19-43 years; mean age, 28.3 years), who were followed up as outpatients in 7 dermatology departments in Italy (Bologna, Rome, Genoa, Cagliari, Milan, Florence, and Bari), agreed to take the self-administered IIEF-5.
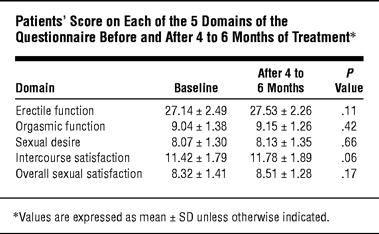
Possible changes in score after 4 to 6 months of treatment vs baseline values were considered within the 5 domains of the questionnaire (partial score) and within each question. Statistical analysis was performed by means of the Wilcoxon test for paired nonparametric data. Table 1 shows the patients’ score on each of the 5 domains of the questionnaire before (at baseline) and after 4 to 6 months of treatment. No changes were statistically significant.
COMMENT
The development of AGA clearly involves a combination of androgens, genetics, and aging. Follicle miniaturization occurs mainly as a result of dihydrotestosterone (DHT). Oral finasteride therapy (1 mg) lowers serum, prostate, and scalp DHT levels and is effective in treating AGA because it inhibits the conversion of testosterone to DHT. Finasteride is a well-tolerated drug, and the only reported adverse effects involve sexual function.
Androgens are known to play an important role in erectile function, and androgen receptors have been identified in cavernosal tissue of rats, although the involvement and precise role of these steroids in humans remains to be established.8-9 Hypothetically, finasteride could cause loss of libido, erectile dysfunction, and decreased ejaculate volume by a reduction in serum and prostate levels of DHT.10-11 Studies have shown that the drug does not affect spermatogenesis or semen production in young men.12
Finasteride-induced sexual dysfunction is nevertheless a benign condition that completely resolves after therapy is discontinued. Sexual dysfunction has also resolved in most men who reported the condition but who continued to take finasteride anyway.3 The drug does not produce significant changes in serum levels of gonadotropins (leutinizing hormone and follicle-stimulating hormone) or in estrogen-testosterone ratios, although testosterone and estrogen levels slightly increase (approximately 15%) during treatment.3, 11
In clinical experience, sexual side effects occur in fewer than 0.5% of subjects who are on a 1-mg regimen of finasteride.4 Trials on the long-term use of 1 mg of finasteride (>4000 men studied in clinical trials for 6 years) show that the incidence of adverse effects does not increase with continuation of treatment.3 However, men with AGA are often severely psychologically affected by their hair loss and therefore can be more susceptible to the psychological impact of possible sexual side effects. Our study using an objective method, the IIEF-5 questionnaire, showed that erectile function of all patients remained stable after 4 to 6 months of treatment with finasteride (1 mg).
Our results support the clinical impression that sexual side effects are actually less common than is reported in clinical trials. This discrepancy may be attributable to the fact that subjects who enrolled in the clinical trials were informed about possible changes in their sexual function and were specifically asked about such changes at every visit, resulting in a higher percentage of reported adverse events than would normally occur. Physicians who prescribe the drug on a daily basis see sexual effects much more rarely, even if they inform their patients about the possible occurrence of these rare side effects. We did not evaluate changes in the ejaculate volume in this study because this subject is not assessed by the questionnaire, although a decrease in ejaculate volume is not rare in our experience.
We believe that the IIEF-5 could be routinely administered to subjects who wish to begin taking finasteride but are particularly worried about sexual side effects. In our experience, the questionnaire is well accepted by patients, who are often anxious about the possible occurrence of sexual side effects and who are eager to be carefully followed up during treatment.